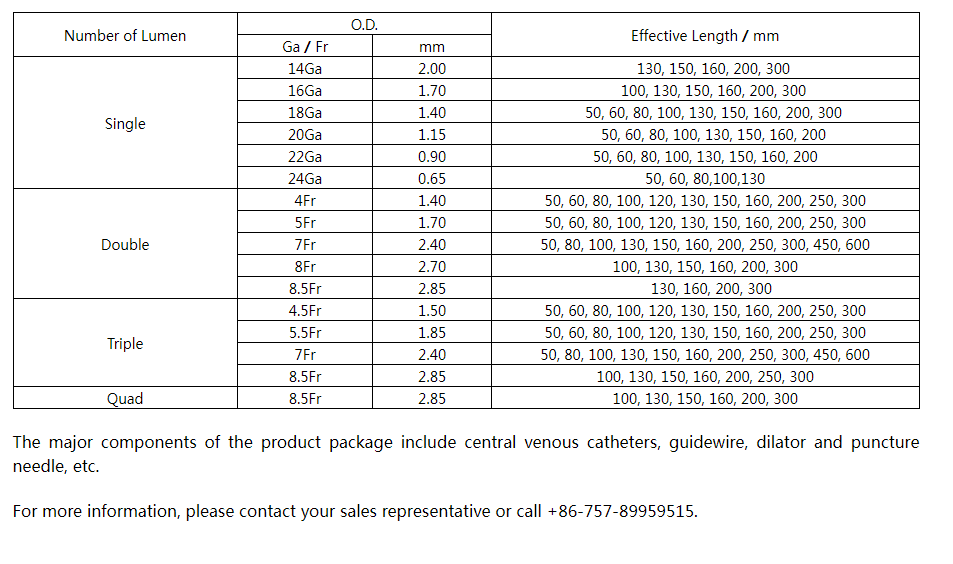
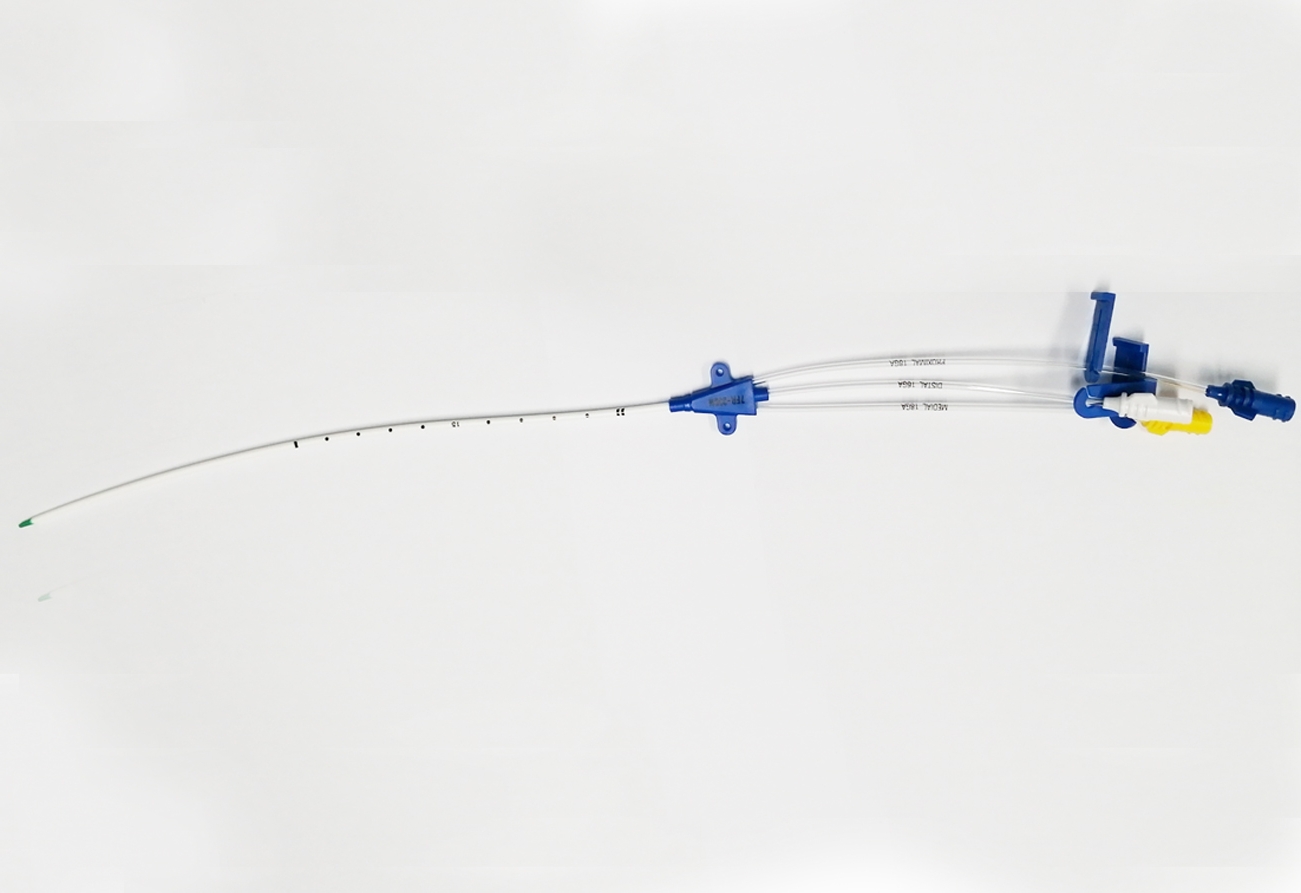
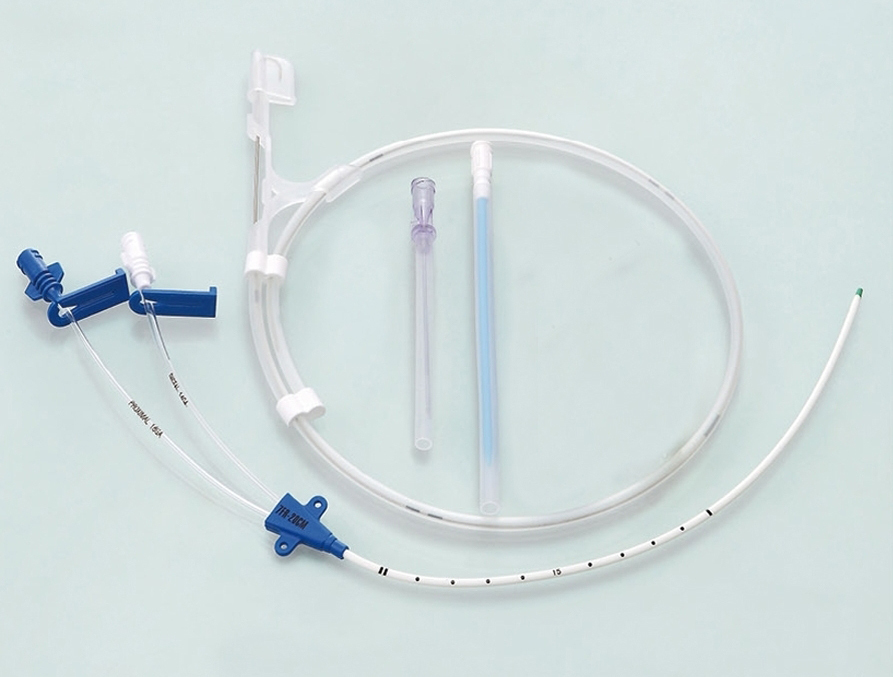
The ABLE® Central Venous Catheters (CVC) are sterile, single-use only polyurethane catheters designed to facilitate infusion therapy in a critical care environment. They are available in variety of lumen configurations, lengths, French and Gauge sizes. The multi lumen variants provide dedicated lumens for infusion therapy, pressure monitoring and venous sampling. The CVC are packaged along with the components and accessories for insertion with Seldinger technique. All products are sterilized by ethylene oxide.
Indication:
Adaptation department:
Main features of the product:
Specification and model:
SSCP
The ABLE® Central Venous Catheters may be applicable to the one of following therapy:
- Monitor of central venous pressure;
- Continuous or discontinuous venous transfusion;
- Blood sampling.
The Catheter is surgically penetrated into three optional puncture points depended on the clinical requirement with Seldinger Technique.
The Insertion Sites are :
1. Internal jugular vein;
2. Subclavian vein;
3. Femoral vein.
It is possible to be inserted inside the body for no more than 30days. If duration exceeds 30 days, it may occur the risk of combining the
catheter and inside tissue, which result in serious incident.
1. Infection or cut wound around the puncture area.
2. Dysfunction of blood coagulation.
3. During the anticoagulant treatment.
4. Symptoms of inadaptability to puncture operation, such as Pneumothorax, vein sclerosis.
5. Abnormal or unclear anatomical situation at the penetration area, such as sever emphysema, obviously inadaptability from previous
operation.
The clinical benefit of the use of CVC’s must be evaluated against the recognized risks and complications of the procedure which include but are not limited to:
1. Infection, necrosis of the puncture point
2. Thrombus
3. Extravasation injury
4. Pheumothorax
5. Bleeding/hematoma
6. Arterial puncture
7. Air embolism
8. Hemothorax
9. Cardiac arrhythmia
10. Occlusion